by Mylène André
Structural studies show that THC cannabinoid inhibits the human enzyme autotaxin
In various European countries and North America, medical cannabis or medicines based on cannabinoids are authorised for therapeutic purposes. While the cannabis plant contains over 100 cannabinoids, THC (D9-tetrahydrocannabinol) and CBD (cannabidiol) are the two best known and characterised constituents.
THC and CBD are administered under different pharmaceutical forms, showing therapeutic effects such as pain and inflammation relief. However, little is known about how THC and other cannabinoids work in the human body at the molecular level.
Based on clinical trials, cannabinoid-containing medications can help to alleviate symptoms of mental disorders such as epilepsy, Alzheimer’s disease, asthma, and cancer, and help prevent weight loss during clinically challenging treatments for AIDS and different forms of cancer.
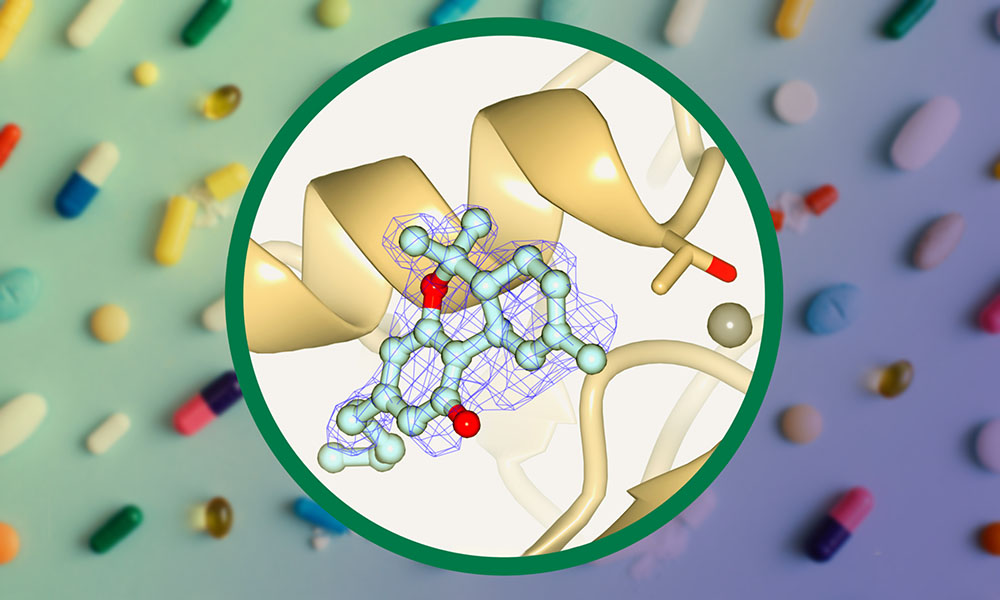
EMBL Grenoble researchers have investigated the interaction between THC and some proteins it might bind to. In a recent study, they showed in vitro that THC inhibits an important human enzyme called autotaxin. This enzyme is involved in many different cellular functions, specifically producing a molecule called lysophosphatidic acid (LPA), which stimulates cell proliferation. A dysregulation of LPA production can lead to the development of cancer, inflammation, or pulmonary fibrosis. Autotaxin is therefore a major target for drug development.
New molecular insights on THC
Understanding how THC and other cannabinoids interact in our cells at the atomic level would help to administer THC more efficiently in therapeutic contexts.
The field of structural biology is particularly relevant to obtain this kind of information. Structural biologists focus on elucidating at the atomic scale the three-dimensional structure of molecules, like proteins or enzymes, and how they interact with each other. These structural results further lead to understanding molecules’ particular function and how to modulate their activities with specific compounds – which are crucial insights to develop effective drugs.
The first step in structural and biochemical studies is to determine how a specific component interacts with molecules in vitro – meaning in the controlled environment of the laboratory – before going for further investigation in vivo, in living organisms.
During their investigation of THC, the McCarthy team obtained the three-dimensional structure of the THC cannabinoid bound with autotaxin. By employing macromolecular crystallography with EMBL’s beamline at the PETRA III synchrotron in Hamburg, they could lay the molecular basis of how THC inhibits this enzyme.
A path to further investigations
Identifying this enzyme as a binding target for THC expands the knowledge on this cannabinoid and provides more data on its possible therapeutic effects at the molecular level and how medical cannabis might contribute to therapy.
“Autotaxin is an essential enzyme in human beings,” said Mathias Eymery, PhD student in the McCarthy team and first author of the publication. “It is responsible for the production of LPA, a major membrane-derived lipid signalling molecule that mediates many different cellular functions. Dysregulations of LPA production by autotaxin are known to have a role in the development of cancer, inflammation, or pulmonary fibrosis.”
In vivo studies are necessary to confirm that the binding between autotaxin and THC is linked to the therapeutic effects of THC administration – as the main known targets of THC in the human body are the CB1 and CB2 cannabinoid receptors, that mediate the psychoactive and pain relieve effects of cannabinoids. Further investigation will help determine the further potential of cannabinoids for medical research and drug development.
SOURCE ARTICLE
Linking medicinal cannabis to autotaxin-lysophosphatidic acid signaling
Eymery M.C, et al.
Life Science Alliance 9 (January 2023)
10.26508/lsa.202201595
This article was first published on EMBL News
