by Mylène André
Recent studies supported by EMBL Grenoble’s expertise in structural biology have identified Altiratinib as a potential drug to stop toxoplasmosis infection and opened up treatment options against malaria
Toxoplasma gondii, Plasmodium falciparum, Cryptosporidium – these parasites of the Apicomplexa family, respectively responsible for toxoplasmosis, malaria and cryptosporidiosis — are still a major issue for public health. Few effective drugs are available, many of which have serious side effects, and immunosuppressed people are particularly susceptible to toxoplasmosis. Drug resistance adds another level of complexity, particularly in the case of malaria, a disease that causes a large number of deaths worldwide.
To tackle these problems, the Institute of Advanced Biosciences (IAB), has led a drug repurposing strategy in collaboration with EMBL Grenoble, targeting the parasite Toxoplasma gondii. The study aimed to identify a potential drug molecule that could be repurposed to stop the infection mechanism, by screening a large library of already approved drugs.
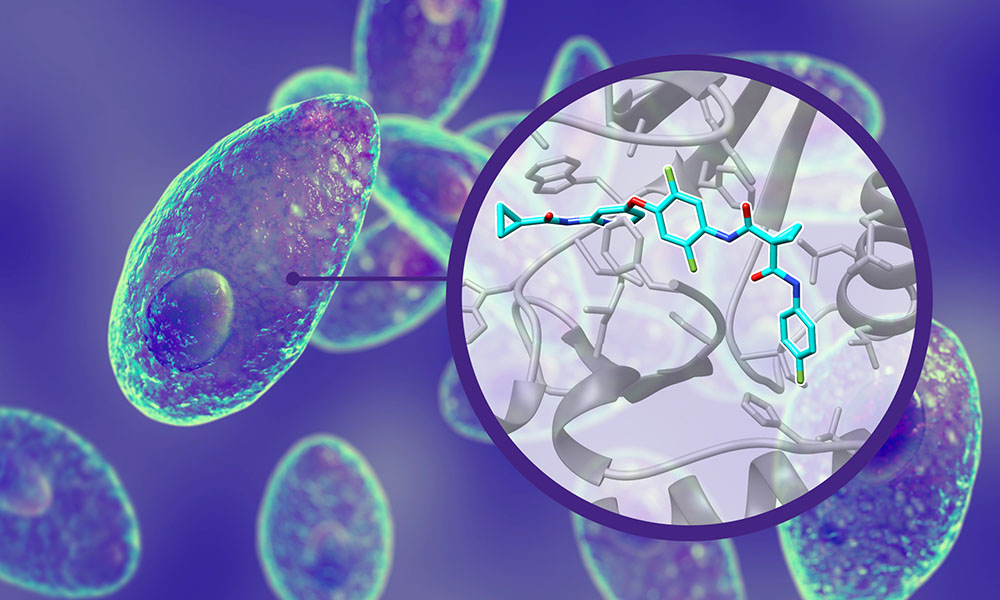
This study, published in Science Translational Medicine, sheds light on how Altiratinib – a drug currently used to treat glioblastoma – perfectly binds a T. gondii protein called PRP4 kinase, preventing the parasite from proliferating inside the human body.
MASSIF-1, the perfect match
Mohamed-Ali Hakimi, a researcher at the IAB and corresponding author of the study, has been working on T. gondii with his team for over 20 years, from reprogramming its sexual stages to studying host-pathogen interactions and looking for effective drugs.
Benefiting from a long-standing collaboration with EMBL Grenoble researcher Matthew Bowler on various aspects of this pathogen and its interactions with the host, and from the complementarity of expertises between IAB and EMBL Grenoble, Hakimi and Bowler initiated studies on available drugs that could be repurposed to block the parasite’s infection mechanisms.
EMBL Grenoble’s expertise in structural biology research and scientific services has been particularly crucial in this drug repurposing strategy. Always at the forefront of technology developments to automate processes in crystallography and structure determination, EMBL Grenoble offers instruments and facilities particularly useful for large-scale drug screening, like the High-Throughput Crystallisation Facility (HTX) and the completely automated beamline MASSIF-1 jointly operated by EMBL and the ESRF.
“The advantage with MASSIF-1 is that we can get the information extremely quickly and easily. Years ago, obtaining a single structure would be the work of a PhD thesis or a much longer project. Christopher Swale, post-doctoral fellow at IAB and first author of this publication, used the HTX technology and MASSIF-1 at EMBL Grenoble, and obtained within two weeks the first structure of the target enzyme showing where the drug binds,” said Matthew Bowler, beamline scientist in charge of MASSIF-1 and one of the authors of the publication.
The rapidity of the automated processes of EMBL’s scientific services allows researchers to try different experimental conditions and optimise settings. “Thanks to this collaboration with EMBL experts, we’re going beyond technical and technological boundaries. We also have enriching exchanges about biological questions to see how we can make improvements,” said Hakimi.
Finding the perfect fit against Toxoplasma gondii
In this study, the researchers not only identified Altiratinib as a potential blocker of the parasite’s infection but also obtained a high resolution 3D structure that shows how the drug binds to the PRP4 kinase protein of T. gondii.
The added value of identifying where the drug precisely binds to the pathogen protein lies in allowing comparisons with how it binds to the similar version of the protein in humans and if it would affect its function in the same way. This ‘affinity selectivity’ of the drug on the pathogen versus the human target is particularly important to specifically target the pathogen and avoid serious side effects in humans when administering the drug.
In the case of Altiratinib, the study confirms that the molecule has a better affinity selectivity on T. gondii PRP4 kinase over the human PRP4 kinase. This enzyme is involved in the RNA splicing mechanism – an important step in gene expression in which specific segments of RNAs are removed in order for the cell to keep the necessary information to produce proteins. In simple terms, disturbing the splicing function in the parasite stops the infection process.
Identifying treatment options against malaria
This discovery is opening up effective antiparasitic drug treatment options against T. gondii, and by extension, against other apicomplexan parasites including Plasmodium falciparum, which causes Malaria. Even though Altiratinib is less effective on P. falciparum, it could be paired with the molecule TCMDC-135051, found to target the same kinase by a research team from the Medicines for Malaria Venture (MMV) consortium and currently under clinical trials.
In this study, IAB researchers realised through simulations that even though Altiratinib and TCMDC-135051 bind to the same target, they have different mechanisms of action. This could open up the possibility of developing bi-therapy treatment to counter drug resistance in malaria.
IAB and EMBL Grenoble researchers will continue to work on drug repurposing strategies against these parasites. Further experiments will take advantage of a new configuration of the MASSIF-1 beamline with the CrystalDirect technology, allowing the complete automation of room temperature experiments. This new modality, difficult to perform manually, could give additional hints towards potential drug treatments.
In the context of the EMBL’s new programme ‘Molecules to Ecosystems’, EMBL Grenoble is pushing the frontiers of structural biology technology, research, and services with a strong focus on Infection Biology and drug development.
Source article
Swale C., et al.
Science Translational Medicine 3 (August 2022)
This article was first published on EMBL News
