In a nutshell
- platform for production of recombinant euraryotic proteins with inserted synthetic amino-acids developed by EMBL scientists in an international collaboration
- applications include glycoengineering, fluorescence labelling and amino acid crosslinking
- future possibility of custom-design proteins for therapeutic biotechnology and pharmaceutical applications
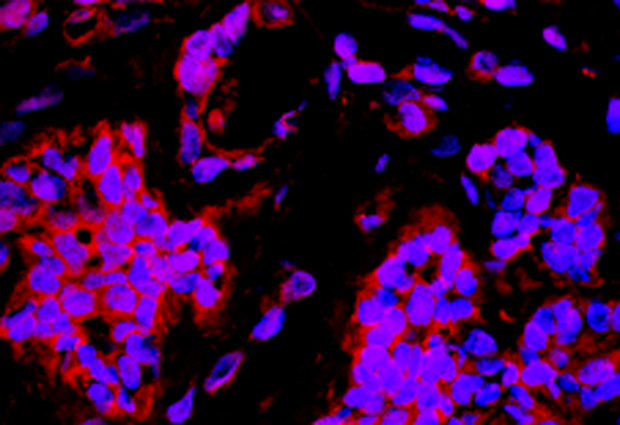
New platform useful for studies of protein binding, human tissue and more
Scientists can now conveniently produce recombinant eukaryotic proteins with synthetic amino-acids inserted at specific locations, thanks to a platform developed by EMBL’s Edward Lemke and colleagues in an international collaboration. The wide range of applications of this new platform, called MultiBacTAG, is demonstrated in a Nature Methods paper published today.
MultiBacTAG’s applications include glycoengineering proteins compatible with human tissue studies, fluorescence labelling of specific targets to measure structure and dynamics in proteins, and amino acid cross-linking in order to map protein binding interfaces.
Lemke anticipates that the platform will provide more insight into how specific protein complexes function, as well as possibilities to custom-design proteins for therapeutic biotechnology and pharmaceutical applications. In their paper, for example, the team used MultiBacTAG to engineer Herceptin – an antibody that associates with cancer cells – to recognise breast cancer cells in human tissue.
To create MultiBacTAG, Lemke’s team combined MultiBac with genetic code expansion (GCE). MultiBac is a recombinant protein production platform previously developed by Imre Berger, formerly a group leader at EMBL. Genetic code expansion (GCE), on another hand, allows scientists to reprogram a protein of interest to incorporate unnatural amino acids at specific sites in its sequence.
With the support of EMBL’s technology transfer arm, EMBLEM, the scientists have submitted a patent application for MultiBacTAG. The platform’s development included a number of EMBL collaborators, including the labs of Martin Jechlinger and Jan Korbel, and the institute’s Genomics Core Facility and Protein Expression and Purification Core Facility.
SOURCE ARTICLE
Koehler C et al. Nature Methods, published online 17 October 2016. DOI: 10.1038/nmeth.4032
This article was originally published on EMBL News
