EMBL scientists develop an important new technique in cryo-electron microscopy
Scientists in the Mahamid group, together with the Théry lab at CytomorphoLab, France, have developed a new technique that allows spatially controlled cell adhesion and the manipulation of cell shapes on cryo-electron microscopy grids. The photo-micropatterning technique – hailed as an important advance by the research community – is presented in a paper published in Nature Methods on 18 November.
The pipeline
Cryo-electron tomography is a technique that allows scientists to produce three-dimensional snapshots of a cell, revealing the intracellular molecular landscape at high resolution. Specimens are prepared by growing cells on a thin electron microscopy support grid, rapidly freezing the grid, and then using a focused ion beam in a scanning electron microscope to ablate – or blast away – surplus material, to produce a very thin section called a lamella.
However, only cells that are positioned in the centre of a grid square are available for this thinning process, so ensuring there are enough cells to work with is challenging. According to Mauricio Toro-Nahuelpan – a postdoc in the Mahamid group, and the paper’s first author – in a typical session five to nine cells can be processed, but this number is limited by the availability of cells properly positioned at the grid centre. Using the micropatterning technique, this bottleneck is removed, as the position of cells on the grid can be controlled with a high degree of spatial accuracy.
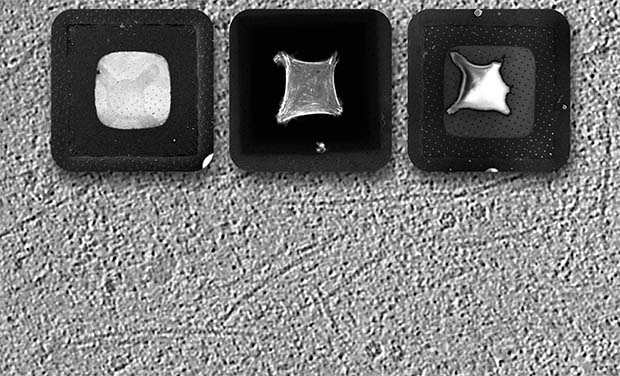
Micropatterning: how it works
Micropatterning of the grids to control the location of cells is achieved by coating standard grids with a passivation layer of polyethylene glycol – essentially, a layer that repels biological material, including cells and proteins. After controlled ablation of the biorepellent area using a UV laser, a micropattern is generated. This biorepellent-free area can be coated with proteins that promote cell adherence at desired locations. Group leader and corresponding author Julia Mahamid explains: “By controlling spatially where you have repelling material versus non-repelling material, you can create areas where proteins and cells will preferentially sit.”
The scientists have demonstrated the technique successfully on a variety of materials, including silicon dioxide, gold and amorphous carbon films. Such films are overlaid on either gold or titanium metal meshes, i.e. grids.
A tool for exploring cell biomechanics
As well as controlling the position of cells on a grid, micropatterning can be used to manipulate the shape of cells, to study their mechanical behaviour. What’s more, numerous different patterns can be generated on the same grid, for direct comparison of different cellular architectures.
“We can pattern very complex shapes, generating very specific configurations of the cell architecture – controlling the cytoskeleton, and intracellular positions of organelles such as the nucleus, Golgi apparatus, and centrosome” says Toro-Nahuelpan. “This opens a new window to explore existing and novel questions in biomechanics, with a resolution that is achievable only via cryo-electron microscopy.”
“Understanding the three-dimensional architecture can help to explain the collective behaviours that give rise to new mechanical properties,” adds Mahamid.
Connecting structural and cell biology
The micropatterning technique is a technical advance with broad implications for cell biology. “It will help to streamline the cryo-electron microscopy pipeline, and facilitate automation of the process,” Toro-Nahuelpan says. It’s also perceived by the research community as a significant bridge between structural and cell biology. There are currently only a handful of labs with the expertise and technology to do cellular cryo-electron tomography, but this latest development is a move towards making this a routine method in the future.
This article was originally published on EMBL News
